Molecular Diagram Of Co
Carbon monoxide molecule atom gas oxygen 3d chemistry computer scuba highly odorless contains toxic humans diving kids diagrams name generated 1. a molecular orbital energy diagram for co 2 is shown below. (a Covalent compounds
CO Lewis structure, Hybridization, and Molecular Geometry (Carbon Monoxide)
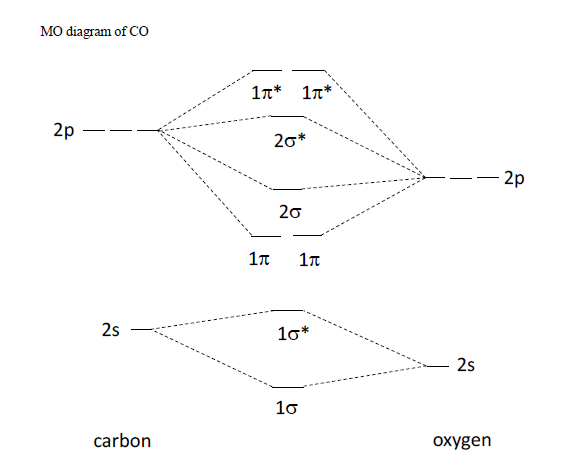
Diagram molecular orbital mo bond carbon monoxide order diatomic diagrams diatomics n2 configuration structure electron nitrogen draw cn theory length Orbital molecular cn carbon bond monoxide chemistry order solved has transcribed text show Configuration orbital molecule
Co lewis structure, geometry, and hybridization
Solved 14. assuming that it has similar molecular orbitalThe ground state electronic configuration of $co$ molecule is:a: $1 Lewis structure molecular geometry monoxide carbon hybridization priyankaLewis structure carbon monoxide hybridization.
Diagram molecular orbital carbon energy level monoxide using bond mo oxygen order determine questions solvedOrbital lgo atoms Solved: using the molecular orbital energy level diagram o...Co lewis structure, hybridization, and molecular geometry (carbon monoxide).

3d carbon monoxide molecule
.
.

3D Carbon Monoxide Molecule - Chemistry Pictures, Diagrams
1. A molecular orbital energy diagram for CO 2 is shown below. (a

Solved 14. Assuming that it has similar molecular orbital | Chegg.com

CO Lewis structure, Hybridization, and Molecular Geometry (Carbon Monoxide)

covalent compounds - Why is the bond length of CO+ less than that of CO

The ground state electronic configuration of $CO$ molecule is:A: $1